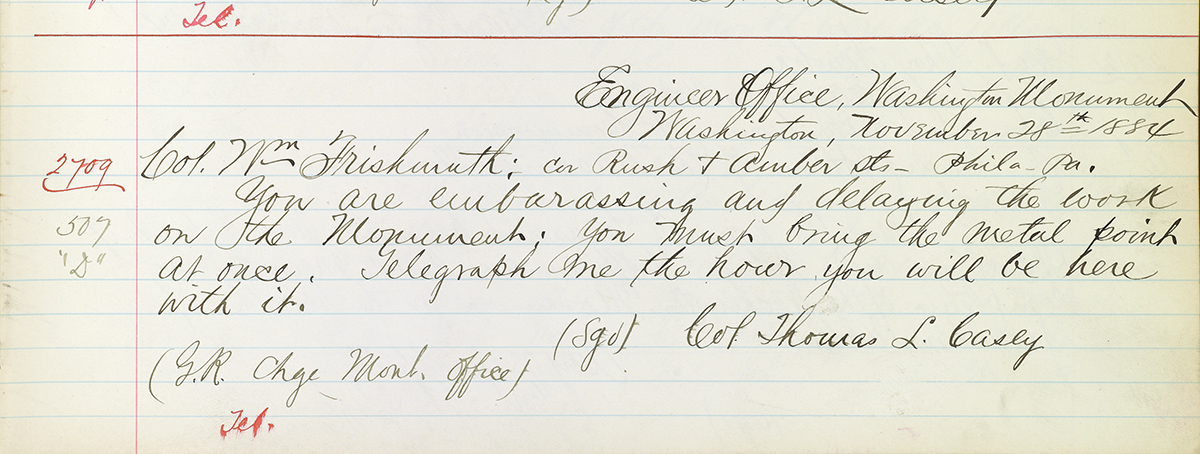Topping Off the Tip
How Aluminum Found Its Way onto the Washington Monument
Summer 2014, Vol. 46, No. 2
By John Lockwood
The recent repairs on the Washington Monument are complete. Gone is the scaffolding that made it look more like a very tall chicken coop than a national shrine to the “father of our country.” Once again, tourists are ascending the 555-foot structure to get a bird’s-eye look at the center of their nation’s capital.
Visitors cannot go all the way to the top, just to 500 feet, but if they could, they would find a tip made of aluminum. Things made of aluminum are commonplace now, but when the monument was completed in 1884, the substance was considered a precious metal.
Construction on a monument to honor our first President began in 1848 but was suspended between 1854 and 1877 because of funding problems and the Civil War. The reopening of the monument this spring came after a 32-month shutdown to repair damages caused by a 2011 earthquake, a shutdown symbolized by scaffolding made of steel—and aluminum.
Aluminum is one of the most common elements in the earth’s crust, after silicon and oxygen. It does not occur in pure form in nature, however. It took decades to learn how to extract aluminum from its ore. Its most important ore is bauxite, named for the French castle Les Baux, in Provence, where it was first discovered.
In 1807, British scientist Sir Humphrey Davy was the first to suggest that the com-pound alumina, named for its presence in alum, contained a metal element. In this, Davy was correct, for alumina is aluminum oxide, or Al2 O3. He also came up with the name for the new metal, aluminum. (Later on, most of the world came to spell it alu-minium, though not the United States.)
Over the years, scientists learned more about aluminum, and the privileged were quite taken with it. Napoleon III, for one, used it to display his wealth—the emperor of the French owned aluminum tableware, used only for the most important guests. His son also had an aluminum baby rattle.
New discoveries gradually lowered the price, but even by 1884, the year of the Washington Monument’s completion, aluminum was still $1.10 an ounce, twice the price of silver.
Aluminum Is Chosen For Tip of Monument
How did Washington, D.C.’s most prominent landmark end up with an aluminum tip? By October 1884, after many years of delay, the Washington Monument was almost finished.
The official in charge, Lt. Col. Thomas Lincoln Casey, wanted to have a metal tip to complete the monument, of either bronze or brass plated with platinum. The man Casey chose for the job was William Frishmuth of Philadelphia.
Frishmuth was a German chemist who had immigrated to the United States. He had even been a student of the German scientist Frederick Wohler. Frishmuth had spent some 28 years and $53,000 experimenting for a way to purify aluminum. The process he hit upon was to heat the ore until the alumina vaporized, then add sodium vapor.
Once he had the assignment safely in hand, Frishmuth bombarded Casey’s office with letters and telegrams about using aluminum. In these messages, by the way, Frishmuth used the alternative spelling, “aluminium.”
On October 25, 1884, Frishmuth wrote to Casey with two proposals about the bronze/brass tip. First, make the tip of an aluminum/bronze alloy, to be plated with gold, which does not tarnish. Or, second, “have the pyramid cast in Aluminium and nicely polished.”
Frishmuth added that the aluminum tip would not tarnish and was also a good conductor of electricity for any lightning-rod system. Casey chose the aluminum tip.
Six days later, on October 31, 1884, Frishmuth telegraphed Casey with two more things to say. One, he would have to use a cast-iron mold rather than a sand mold for the monument tip. Two, although he would indeed try to cast the tip of aluminum, just in case, he would also cast another one, of 90 percent copper and 10 percent aluminum. This alloy was known at the time as “aluminum gold.” It already had a pale gold color, but Frishmuth would gild it anyway.
Frishmuth’s momentary lack of confidence was unnecessary. Sometime in November 1884, Frishmuth successfully cast the aluminum tip.
Frishmuth Puts Tip on Display in Tiffany’s
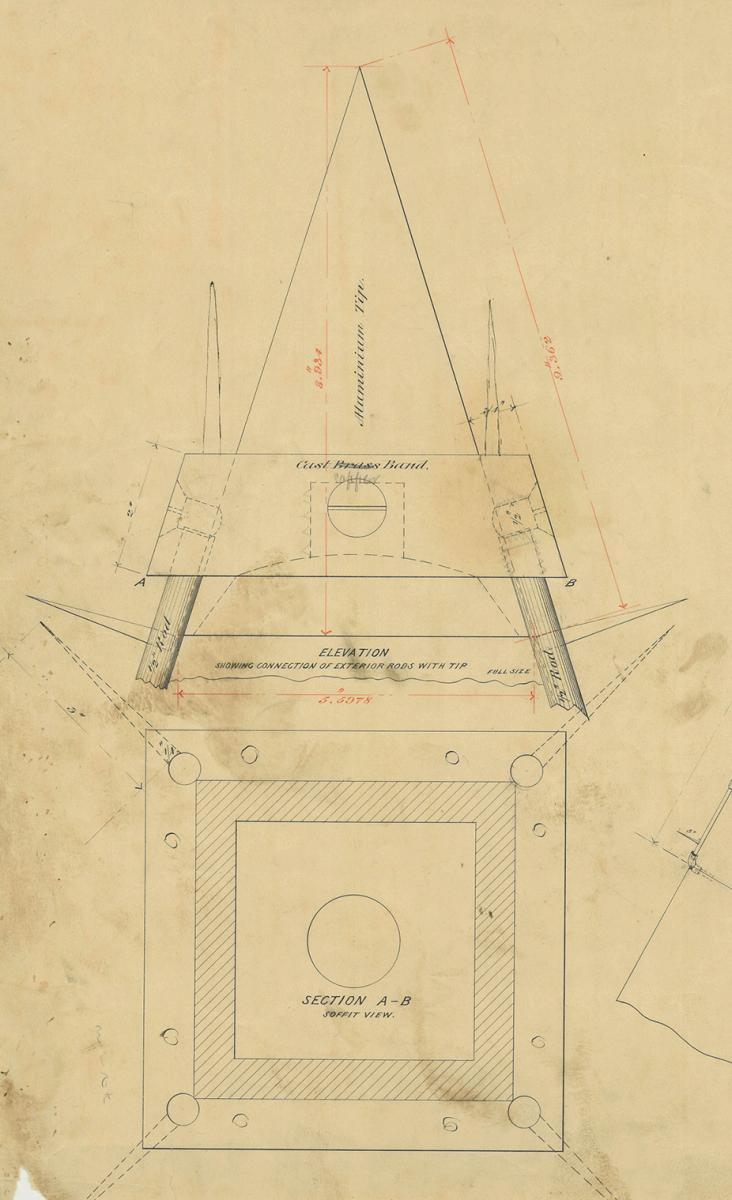
It was the largest piece of aluminum cast up to that time, at 8 inches tall and 100 ounces. Frishmuth charged $225.00 for it, higher than the earlier agreed-upon price of $100.00, necessitating a further exchange of telegrams.
Before sending it on to Washington, Frishmuth put it on display at Tiffany’s jewelry store in New York City—without permission. On November 25, Frishmuth even sent a telegram to Capt. George Davis, Casey’s assistant, telling him about the display. There were “thousand [sic] of persons see the pyramid given high credit to all of us.”
Some stern messages from Washington persuaded Frishmuth to send the tip without further delay. On November 28, Casey sent Frishmuth the following message: “You are embarassing [sic] and delaying the work on the Monument. You must bring the metal point at once. Telegraph me the hour you will be here with it.”
A bit later that same day, Frishmuth also got the following from Casey: “You cannot keep that point in Philadelphia any longer. Must send or bring it on this afternoon. You are embarassing [sic] and damaging the Government.”
Frishmuth lost no time. On November 28, he telegraphed Davis that the tip would be in the office the next day. On November 29, Casey responded: “The point is received and is satisfactory in every way. Vouchers sent you by mail.” Rather than leave well enough alone, on November 29 Frishmuth telegraphed right back to Casey, expressing thanks—and asking him if the tip could be exhibited “in the Capitol Senate & Congress Monday.” Monday was December 1 that year. There was no exhibit.
Also on December 1, Frishmuth wrote Casey, with the vouchers signed, plus “I thank you a thousand times for your kindness, in the honor you have given me.”
The aluminum tip was finally put into place during a ceremony on December 6, 1884.
A Discovery Is Made: Tip Isn’t All Aluminum
Just six days later, on December 12, Casey received another handwritten message from Frishmuth. Professor F. I. Ricardo Seaven of Philadelphia had examined the tip on November 25 for Frishmuth and determined that it was actually 97.75 percent aluminum. The remainder was 1.70 percent iron, and 0.55 percent silicon. Casey’s response, if any, does not seem to have been recorded—not in the National Archives, anyhow.
As for Frishmuth, he had some aluminum left over from casting the tip, so he advertised an aluminum watch charm in the February 28, 1885, Scientific American. An aluminum charm would cost 75 cents, an aluminum alloy charm 30 cents, and the alloy with gilding also at 30 cents.
Frishmuth was also not quite done with the Washington Monument.
On June 9, 1885, after recent lightning strikes on the monument, he wrote to Casey with advice about a lightning-rod system, and he offered to provide aluminum points for it, an offer Casey did not take up. Instead, Casey hired another Philadelphian for the job, Joseph Neumann. Casey went to Philadelphia, on June 12, 1885, to hire Neumann in person.
Frishmuth still wanted to keep some of the precise details of his now-famous process a secret, but one of his employees tried to steal those secrets. Also on June 9, 1885, while Frishmuth was writing his letter to Casey, Charles Krug, who had been working for Frishmuth as an engineer, was convicted in Philadelphia of stealing $2.50 worth of chemical supplies in an attempt to learn Frishmuth’s exact process.
By now, lightning rods or no lightning rods, both Casey and Davis found Frishmuth a bit tiresome. For instance, in one letter to Casey, dated October 16, 1885, Davis sarcastically calls Frishmuth “our mutual friend.”
In a June 15, 1885, letter to a A. Mordecai, also of Philadelphia, Casey looked back on it all and concluded that aluminum was not a practical metal for widespread use just yet. Although aluminum did have its advantages, Casey cited the difficulty in getting even a 100-ounce sample; the troubles with Frishmuth, including the display at Tiffany’s; and the last-minute increase in Frishmuth’s price from $100 to $225. All this “would seem to imply that Alumininum [sic] cannot yet be manufactured at such rates as to make it a commercial success.”
A Method Is Found for Cheap Aluminum
But there was a young student who would soon make it a commercial success. On February 23, 1886, less than two years after the Washington Monument’s completion, a recent graduate from Oberlin College, Ohio, by the name of Charles Martin Hall discovered the purification process that made aluminum cheap and commonplace.
Sometime in 1883, Hall was attending the class of a chemistry professor, Frank F. Jewett, who told his students that anybody who could convert alumina to aluminum, cheaply and in quantity, would make a fortune. Something about this took hold of young Hall and would not let go.
He could not afford a regular laboratory, so he constructed a wooden shed behind his parents’ house to serve as one. He could not even afford electric batteries, so he made them himself. Hall decided to use electrolysis as his method. The trouble was that alumina melts at 2,050 degrees Celsius, and his furnace and bellows (both of which he also made himself ) could not handle that. So he tried various compounds, which, when molten, would dissolve the alumina and enable the electrolysis to work.
On February 23, 1886, he finally hit upon cryolite from Greenland as a medium, running electricity through the cryolite and alumina for two hours. After it was all over, he ran with his samples to Professor Jewett.
Unlike some inventors, Hall proved to be good at business as well. Sometime in 1888, he came to Pittsburgh and helped found the Pittsburgh Reduction Company. In December of that year, the company opened for business. Hall eventually made a fortune of $27 million. Coincidentally, a young Frenchman named Paul Heroult was working on the same process. He succeeded in purifying aluminum just two months after Hall.
As for Frishmuth’s aluminum tip, it has been in place ever since. It nearly disappeared in July 1941, when the idea got about that it should be donated to a national aluminum scrap drive. Collecting used aluminum for civilian recycling would free up virgin aluminum for building military airplanes. Fortunately, the idea to decapitate the monument was quietly dropped.
So, ever since 1884, the tip has been on duty, overseeing Washington.
During monument restorations in 1934, 1964, and 1998, the Frishmuth tip was found to be in good shape, and still untarnished. Even now, on sunny days it can sometimes be seen from the ground, glowing like a small white point.
Today, aluminum is so common, it is used almost everywhere—even for scaffolding.
John Lockwood is a native Washingtonian who has published some 80 articles in newspapers and magazines since 2002. He and his late brother Charles also wrote a book for Oxford University Press, The Siege of Washington, about the nearly defenseless condition of Washington, D.C., during the first 12 days of the American Civil War.
Note on Sources
Records about the building of the Washington Monument used in this article are in entry 492, box 8, items 2867, 2871, 2884, 2889, 2890, 2891, 2898, and 3078; entry 495, volume 4, pages 23–25, items 2706, 2707, 2708, and 2709, and, pages 145–146 of items 2915, 2916, 2917, and 2918; all in Records of the Joint Commission for the Completion of the Washington Monument, 1876–1892, Records of the Office of Public Buildings and Public Parks of the National Capital, Record Group 42, National Archives Building, Washington, D.C.
Charles Martin Hall’s method for refining pure aluminum from its ore, devised in 1886, led to a 50th-anniversary celebration in the media outlets of 1936. I found four such examples. The earliest was in the January 29, 1936, Washington Post, on page 8, “The Post Impressionist. From Rare to Common.” The others were all on February 23, 1836. One was “Aluminum’s Birthday,” in the New York Times on page E-8. Another was also in the New York Times, on page 22, “Honor Aluminum Expert.” Finally, I also found in the New York Times an article named “The Week in Science: The Romance of Aluminum,” on page XX6.
In 1964, the entire Washington Monument was restored. The aluminum tip was found to be in good shape. See “Cap on Monument in Capital Tested,” in the New York Times, August 30, 1964, on page R-1.
An article in the Washington Post makes William Frishmuth’s assignment by Casey seem smooth and easy, in “Sure, Frishmuth Said He Could Make It and There It Is Atop the Monument,” in the Washington Post of August 21, 1964, on page B-1. Not quite so easy as that.
In 1885, Frishmuth’s process was still the best, cheapest way to refine any aluminum at all, as shown in the flattering article, “New Processes for Aluminum,” in The Manufacturer and Builder: A Practical Journal of Industrial Progress, on January 1, 1885, page 15. Similar flattery may be found “Manufacture of Aluminum,” in American Druggist for March 1, 1885, page 53; and “The Secret of Making Cheap Alumina,” in the Evening Star, of Washington D.C., for June 13, 1885, page 2; and even in an advertisement offering watch charms made with 2% aluminum, in Scientific American, for February 28, 1885, page 140.
All this would change with Hall’s discovery in 1886, as told in “Plentiful in Clay,” in the Pittsburg Dispatch for September 17, 1889, on page 2, and “City Briefs,” in the Washington Post for February 22, 1918, page 2.
The story of how the monument nearly lost its tip to an aluminum scrap drive is told in “To ‘Scalp’ Washington Monument,” New York Times, July 11, 1941, page 11.
A quite prescient article foresees the importance of Hall’s process for civilization, in “Aluminum—The Metal of the Future,” in The Cosmopolitan: A Monthly Illustrated Magazine, for January 1892, on page 277. Their picture of an aluminum-laden future was on target.